


xxxxxThe German
physicist Wilhelm Roentgen discovered X-rays in November 1895 while experimenting with
cathode rays. Although he had blocked off the rays being emitted
from the Crookes tube, using a sheet of thin card, he suddenly saw
that a piece of paper coated with a luminescent substance was
giving off a glimmer of light. After further research he concluded
that the tube was emitting invisible rays capable of penetrating
not only card, but also wood and a thin layer of steel. He
produced a paper on his find in January 1896, and within a few
days this amazing discovery was known throughout the world. Its
enormous value was quickly realised, not only in the field of
medicine - where it could provide a picture of broken bones
and vital organs - but also in other branches of science. For
this discovery he was awarded the first Nobel Prize for physics in
1901. During his career, serving as professor of physics at
Giessen, Wurzburg and Munich Universities, he also made valuable
contributions in the study of mechanics, electricity and heat.
WILHELM ROENTGEN 1845 -
1923 (Va, Vb,
Vc, E7, G5)
Acknowledgements
Roentgen: drawing
by the artist Walter E. Hodgson, 1896, for The
Windsor Magazine, a monthly illustrated journal,
published in London from 1895 to 1939. X-ray:
print, presented to the Swiss physicist Ludwig Zehnder (1854-1949),
professor of physics at the University of Freiburg, Germany, in
January 1896. Lenard: 1900, photographer
unknown – Emilio Segrè Visual Archives, American Institute of
Physics, College Park, Maryland. Birthplace:
date and photographer unknown. Becquerel:
date and photographer unknown, contained in the New Catholic
Dictionary, 1910. Diagram: by courtesy
of Explain That Stuff.
 xxxxxIt was in November
1895, while experimenting with cathode
rays, that the German physicist Wilhelm Roentgen discovered the
highly penetrating radiation which came to be known as X-rays.
This momentous discovery proved of vital practical importance as a
diagnostic aid, not only in the field of medicine - where its
enormous potential was quickly realised - but also in a
number of other areas, including engineering for the testing of
metals, and physics for determining crystal structures and
analysing complex mixtures of elements.
xxxxxIt was in November
1895, while experimenting with cathode
rays, that the German physicist Wilhelm Roentgen discovered the
highly penetrating radiation which came to be known as X-rays.
This momentous discovery proved of vital practical importance as a
diagnostic aid, not only in the field of medicine - where its
enormous potential was quickly realised - but also in a
number of other areas, including engineering for the testing of
metals, and physics for determining crystal structures and
analysing complex mixtures of elements.
xxxxxRoentgen
was born in Lennep, Prussia, but with the coming of 1848, the Year
of the Revolutions, the family moved to Apeldoom in the
Netherlands (his mother being of Dutch descent), and it was here
that he spent his childhood. He attended a private boarding school
until 1861, and then studied at the technical college at Utrecht
before taking a course in mechanical engineering at Zurich
Polytechnic. Itxwas here that he was
taught by August Kundt (1839-1894),
the German physicist who made important advances in the study of
sound and light. Kundt awakened his interest in physics, and after
Roentgen had obtained a doctorate for his study of gases, he
worked alongside Kundt as his assistant, first at Wurzburg
University and then at the newly formed University of Strasbourg.
xxxxxIn 1874 he
was appointed lecturer at Strasbourg and then, after serving as
professor of physics and mathematics at the Agricultural Academy
of Hohenehim, he returned to Strasbourg in 1876 to work once again
alongside Kundt as an associate professor. Three years later he
took up the chair of physics at the University of Giessen in
Germany, and his experiments there, conducted over a period of
eight years, confirmed his place as one of the leading German
physicists. As a result, he was appointed professor and director
of the Physics Institute at Wurzburg University in Bavaria in 1888
and elected Rector in 1894.
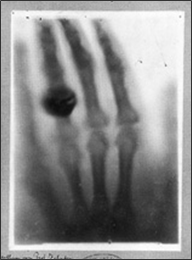 xxxxxIt was on the evening of the 8th
November the following year, while
studying the luminescence that cathode rays produced when passed
through a variety of gases, that Roentgen stumbled upon a new,
penetrating ray. Working in a darkened room, he suddenly observed
that a sheet of paper coated with a luminescent substance called barium platinocyanide - lying some nine
feet away - was giving off a glimmer of light. This mystified
him because, as part of the experiment he was conducting at that
time, he had deliberately blocked off the cathode rays being
emitted from the Crookes tube (the forerunner of the cathode ray
tube) with a sheet of thin black card. He then carried out further
experiments and was forced to conclude that the tube was emitting
invisible rays capable of penetrating a thick sheet of card, a
piece of wood, photographic plates and even a thin layer of metal.
And to prove the value of this new form of radiation he persuaded
his wife Bertha to let him take an X-ray photograph of her
left hand. After a 15 minute exposure she provided the first
radiograph of a human being (here illustrated).
xxxxxIt was on the evening of the 8th
November the following year, while
studying the luminescence that cathode rays produced when passed
through a variety of gases, that Roentgen stumbled upon a new,
penetrating ray. Working in a darkened room, he suddenly observed
that a sheet of paper coated with a luminescent substance called barium platinocyanide - lying some nine
feet away - was giving off a glimmer of light. This mystified
him because, as part of the experiment he was conducting at that
time, he had deliberately blocked off the cathode rays being
emitted from the Crookes tube (the forerunner of the cathode ray
tube) with a sheet of thin black card. He then carried out further
experiments and was forced to conclude that the tube was emitting
invisible rays capable of penetrating a thick sheet of card, a
piece of wood, photographic plates and even a thin layer of metal.
And to prove the value of this new form of radiation he persuaded
his wife Bertha to let him take an X-ray photograph of her
left hand. After a 15 minute exposure she provided the first
radiograph of a human being (here illustrated).
xxxxxIn January
1896 Roentgen read out a paper before the Physico-Medical
Society of Wurzburg. Accepting the existence of these rays, but
not understanding their make up, he used the term X-rays
because in mathematics this letter is used to indicate the
unknown. In the long term this became the generally accepted name,
though in some countries, including Germany, they are still known
as Roentgen Rays. He followed up this
meeting with the publication of three papers entitled On
a New Kind of Rays. The news of this fascinating
discovery spread across the world in a matter of days. The idea
that there existed a kind of invisible light that could penetrate
wood and show the bones of a man’s hand was almost unbelievable.
The London Standard, printing the news
on the 7th January felt it necessary to assure its readers that
“there is no joke or humbug in the matter. It is a serious
discovery by a serious German professor”.

xxxxxAs one
would expect, honours were showered upon Roentgen from across the
world, and in January 1896 he travelled to Berlin to present his
work to Emperor Wilhelm II. In the same year he received the
Rumford Medal of the Royal Society of London. Hexshared
this medal with the German Philipp
Lenard (1862-1947) (illustrated), a physicist who
had also carried out extensive research into cathode rays and
produced a much-improved vacuum tube to assist in this work.
xxxxxIn 1900
Roentgen was appointed professor of the new Physics Institute at
the University of Munich. After the First World War, with
inflation spiralling out of control, he struggled to make ends
meet and was eventually declared bankrupt. He retired in 1920, a
year after Bertha’s death, and died three years later of stomach
cancer. Regarded today as the father of diagnostic radiology, he
was buried alongside his wife and parents in a cemetery at
Giessen.
xxxxxDuring a
lifetime devoted to science, Roentgen made a major contribution,
to knowledge, particularly in the field of physics. He made
advances in mechanics, electricity and heat, conducting research
on a wide range of topics, including elasticity, the specific heat
of gases, the capillary action of fluids, the conduction of heat
in crystals, and polarized light.
xxxxxIncidentally, for reasons which are not clear Roentgen stated in
his will that upon his death all his personal and scientific
correspondence was to be destroyed. During his lifetime he refused
to make any money out of his findings. He argued that any advances
that were the result of scientific research belonged to humanity,
and should be freely available to all. He also opposed the use of
the term Roentgen Rays, but today the unit of radiation exposure
is known universally as the roentgen. ……
 xxxxx…… It
is very likely that many scientists encountered X-rays in the
course of their experiments but failed to identify them. It is
known that the English physicist William Crookes often complained
that he found photographic plates were foggy or darkened on taking
them out of their boxes. This was almost certainly due to their
exposure to X-rays during his experiments! ……
xxxxx…… It
is very likely that many scientists encountered X-rays in the
course of their experiments but failed to identify them. It is
known that the English physicist William Crookes often complained
that he found photographic plates were foggy or darkened on taking
them out of their boxes. This was almost certainly due to their
exposure to X-rays during his experiments! ……
xxxxx…… As we have seen, the American inventor Thomas Edison
played an important part in the development of the X-ray. In
1895 he made a study of materials which had the ability to
fluoresce, and discovered that calcium tungstate was the best
substance to use. This finding stepped up the use of the X-ray,
particularly in its medical usage. X-rays were being used in
the United States as early as 1896, and were soon employed in many
countries to assist in dealing with bone fractures and gunshot
wounds. ……
 xxxxx…… Andxin
1897 the English scientist Joseph John
Thomson (1856-1940) showed that
cathode rays were electrons (he called them “corpuscles”) -
subatomic elementary particles that carry a negative electric
charge and are an integral part of matter. ……
xxxxx…… Andxin
1897 the English scientist Joseph John
Thomson (1856-1940) showed that
cathode rays were electrons (he called them “corpuscles”) -
subatomic elementary particles that carry a negative electric
charge and are an integral part of matter. ……
xxxxx…… The house in Lennep where Roentgen was born (illustrated), situated 25
miles east of Dusseldorf, is now a museum dedicated to his life and
work.
Vc-1881-1901-Vc-1881-1901-Vc-1881-1901-Vc-1881-1901-Vc-1881-1901-Vc-1881-1901-Vc
Including:
Henri
Becquerel

xxxxxA few months
after Wilhelm Roentgen’s discovery of X-rays, the French
physicist Henri Becquerel
(1852-1908) discovered the phenomenon known as radioactivity
- the spontaneous emission of radiation by a material. In
1886, after a lengthy study of luminescent materials, he found,
virtually by chance, that uranium atoms, present in the salts he
used in his experiments, emitted an entirely new kind of
radiation. He studied further this radioactivity, and concluded
that the atom had an internal structure and was not, in fact, the
ultimate particle of matter. Research into radioactivity was then
taken up by others. As we shall see, Pierre and Marie Curie found
other radioactive materials in 1898 - polonium and radium - and a year later
the New Zealand physicist Ernest Rutherford began his research
into the disintegration of the elements and the chemistry of
radioactive substances. Becquerel’s discovery, revolutionary in
the extreme, paved the way for the study of nuclear physics, and
marked the beginning of the nuclear age. For his research into
spontaneous radioactivity he and Pierre and Marie Curie shared the
Nobel Prize for Physics in 1903.
 xxxxxA few months after the discovery of X-rays, the
French physicist Henri Becquerel (1852-1908), taking Roentgen’s findings a step
further, discovered - again by chance rather than design -
the phenomenon known as radioactivity, the spontaneous emission of
radiation by a material. In time
this discovery, examined further, was to force scientists
the world over to reassess their views on the structure of the
atom, paving the way for the study of nuclear physics and the
beginning of the nuclear age.
xxxxxA few months after the discovery of X-rays, the
French physicist Henri Becquerel (1852-1908), taking Roentgen’s findings a step
further, discovered - again by chance rather than design -
the phenomenon known as radioactivity, the spontaneous emission of
radiation by a material. In time
this discovery, examined further, was to force scientists
the world over to reassess their views on the structure of the
atom, paving the way for the study of nuclear physics and the
beginning of the nuclear age.
xxxxxBorn in
Paris in 1852, Becquerel came from a family of physicians, the
most notable being his grandfather Antoine-César Becquerel (1788-1878), one of the founders of
electrochemistry, and his father, Alexandre-Edmond Becquerel
(1820-91), the inventor of the phosphoroscope. Becquerel
received his scientific training at the École
Polytechnique in Paris and
then studied engineering at the Bridges and Highways School in the
city’s suburbs. He graduated in 1877 and worked for many years as
an engineer in the Department of Bridges and Highways, ending his
career as chief engineer in 1894. As a physicist he taught at the
École Polytechnique and was elected to
the Academy of Sciences in 1889. He was appointed professor of
physics at the Museum of Natural history in 1892, and at the École Polytechnique three years later. His
early research centred around polarized light and infrared
radiation, and he also continued his father’s research into
phosphorescence and fluorescence.
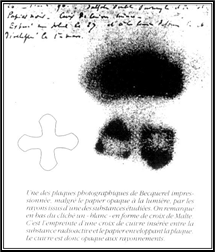 xxxxxEarly in 1896, intrigued by Roentgen’s experiments
and already possessing a considerable knowledge concerning the
emission of fluorescence, he began a study of luminescent
materials in order to find out whether they emitted X-rays.
Wrapping some photographic plates in black paper and putting the
crystals of a fluorescent chemical on top - potassium
uranyl sulphate - he placed the package in bright
sunlight. On opening the packet several hours later he found that
there was a greyish area on the plates. He concluded that the
sun’s ultra-violent rays had induced fluorescence, and that
the X-rays contained within had penetrated the black paper
and “fogged” the plates. The following month, however, having left
a second package, topped again with potassium
uranyl sulphate, in a closed drawer - well shielded
from any ultra-violet rays - he discovered to his
surprise that the plates showed sharp outlines of the mineral
samples. It was clear that fluorescence had played no part in this
transfer. At this stage he concluded that salts of uranium were
“particularly active”, but by May 1896, following further
experiments, his discovery was complete, and he could confirm his
findings. The new rays emerged from the element uranium. (The
illustration shows one of the plates fogged by exposure to
radiation from the uranium salts. The shadow of a metal Maltese
cross, placed between the plate and the salts, is clearly
visible).
xxxxxEarly in 1896, intrigued by Roentgen’s experiments
and already possessing a considerable knowledge concerning the
emission of fluorescence, he began a study of luminescent
materials in order to find out whether they emitted X-rays.
Wrapping some photographic plates in black paper and putting the
crystals of a fluorescent chemical on top - potassium
uranyl sulphate - he placed the package in bright
sunlight. On opening the packet several hours later he found that
there was a greyish area on the plates. He concluded that the
sun’s ultra-violent rays had induced fluorescence, and that
the X-rays contained within had penetrated the black paper
and “fogged” the plates. The following month, however, having left
a second package, topped again with potassium
uranyl sulphate, in a closed drawer - well shielded
from any ultra-violet rays - he discovered to his
surprise that the plates showed sharp outlines of the mineral
samples. It was clear that fluorescence had played no part in this
transfer. At this stage he concluded that salts of uranium were
“particularly active”, but by May 1896, following further
experiments, his discovery was complete, and he could confirm his
findings. The new rays emerged from the element uranium. (The
illustration shows one of the plates fogged by exposure to
radiation from the uranium salts. The shadow of a metal Maltese
cross, placed between the plate and the salts, is clearly
visible).
xxxxxArmed with
this information, Becquerel then began to study this new
radiation, and by 1899 had discovered that, whilst it was similar
to X-rays, it could be deflected by a magnetic field. This
led him to the conclusion that part of it at least was made up of
tiny charged particles, and that these electrons were coming from
the atoms of uranium which had formed part of his fluorescent
compound. That being the case, it was evident - revolutionary
though it was - that the atom had an internal structure and
was not the ultimate particle of matter.
xxxxxAs we shall
see, research into radioactivity was then taken up by others. The
French physicists Pierre and Marie Curie,
for example, went in search of other radioactive materials, and
this led to the discovery of polonium and radium in 1898 - both found in
uranium ores. A year later the New Zealand physicist Ernest
Rutherford, who
became known as the father of nuclear physics and the founder of modern atomic
theory, showed that radioactive material emits more than one kind
of ray. He was awarded the Nobel Prize in Chemistry in 1908 “for
his investigations into the disintegration of the elements, and
the chemistry of radioactive substances.”
 xxxxxIn 1900 Becquerel isolated electrons in radiation
and, the following year, he was the first to prove the phenomenon
of radioactive transformation. And in 1901 his report of a burn,
suffered when he carried an active sample of radium in his coat
pocket, eventually led to the use of radium in medical treatment!
His two major works were Research on Phosphorescence, and Discovery
of the Invisible Radiation Emitted by Uranium. He was
elected president of the French Academy of Sciences, and for his
discovery of spontaneous radioactivity he shared the 1903 Nobel
Prize for Physics with Marie and Pierre Curie. He died at Le
Croisic, a small fishing port in Brittany, in August 1908, aged
55.
xxxxxIn 1900 Becquerel isolated electrons in radiation
and, the following year, he was the first to prove the phenomenon
of radioactive transformation. And in 1901 his report of a burn,
suffered when he carried an active sample of radium in his coat
pocket, eventually led to the use of radium in medical treatment!
His two major works were Research on Phosphorescence, and Discovery
of the Invisible Radiation Emitted by Uranium. He was
elected president of the French Academy of Sciences, and for his
discovery of spontaneous radioactivity he shared the 1903 Nobel
Prize for Physics with Marie and Pierre Curie. He died at Le
Croisic, a small fishing port in Brittany, in August 1908, aged
55.
xxxxxIncidentally, in 1903 Becquerel supervised the work of Marie
Curie when she was working for her doctorate at the University of
Paris. ……
xxxxx…… The SI unit for radioactivity, the becquerel,
is named after the French physicist, and he also has craters named
after him on both the Moon and Mars. ……
xxxxx…… During his career he also researched into the
physical properties of nickel, cobalt and ozone, and made a study
of how crystals absorb light.






 xxxxxIt was in November
1895, while experimenting with cathode
rays, that the German physicist Wilhelm Roentgen discovered the
highly penetrating radiation which came to be known as X-
xxxxxIt was in November
1895, while experimenting with cathode
rays, that the German physicist Wilhelm Roentgen discovered the
highly penetrating radiation which came to be known as X- xxxxxIt was on the evening of the 8th
November the following year, while
studying the luminescence that cathode rays produced when passed
through a variety of gases, that Roentgen stumbled upon a new,
penetrating ray. Working in a darkened room, he suddenly observed
that a sheet of paper coated with a luminescent substance called barium platinocyanide -
xxxxxIt was on the evening of the 8th
November the following year, while
studying the luminescence that cathode rays produced when passed
through a variety of gases, that Roentgen stumbled upon a new,
penetrating ray. Working in a darkened room, he suddenly observed
that a sheet of paper coated with a luminescent substance called barium platinocyanide -
 xxxxx…… It
is very likely that many scientists encountered X-
xxxxx…… It
is very likely that many scientists encountered X- xxxxx…… Andxin
1897 the English scientist Joseph John
Thomson (1856-
xxxxx…… Andxin
1897 the English scientist Joseph John
Thomson (1856-
 xxxxxA few months after the discovery of X-
xxxxxA few months after the discovery of X- xxxxxEarly in 1896, intrigued by Roentgen’s experiments
and already possessing a considerable knowledge concerning the
emission of fluorescence, he began a study of luminescent
materials in order to find out whether they emitted X-
xxxxxEarly in 1896, intrigued by Roentgen’s experiments
and already possessing a considerable knowledge concerning the
emission of fluorescence, he began a study of luminescent
materials in order to find out whether they emitted X-
 xxxxxIn 1900 Becquerel isolated electrons in radiation
and, the following year, he was the first to prove the phenomenon
of radioactive transformation. And in 1901 his report of a burn,
suffered when he carried an active sample of radium in his coat
pocket, eventually led to the use of radium in medical treatment!
His two major works were Research on Phosphorescence, and Discovery
of the Invisible Radiation Emitted by Uranium. He was
elected president of the French Academy of Sciences, and for his
discovery of spontaneous radioactivity he shared the 1903 Nobel
Prize for Physics with Marie and Pierre Curie. He died at Le
Croisic, a small fishing port in Brittany, in August 1908, aged
55.
xxxxxIn 1900 Becquerel isolated electrons in radiation
and, the following year, he was the first to prove the phenomenon
of radioactive transformation. And in 1901 his report of a burn,
suffered when he carried an active sample of radium in his coat
pocket, eventually led to the use of radium in medical treatment!
His two major works were Research on Phosphorescence, and Discovery
of the Invisible Radiation Emitted by Uranium. He was
elected president of the French Academy of Sciences, and for his
discovery of spontaneous radioactivity he shared the 1903 Nobel
Prize for Physics with Marie and Pierre Curie. He died at Le
Croisic, a small fishing port in Brittany, in August 1908, aged
55.

