


xxxxxThe Russian chemist Dmitri Mendeleev is famous above all for his periodic table of chemical elements, compiled in 1869 and improved two years later. Others had classified these elements -
DMITRI MENDELEEV 1834 -
Acknowledgements
Mendeleev: detail, by the Russian painter Ivan Kramskoi (1837-
 xxxxxIt was in March 1869 that the Russian chemist Dmitri Mendeleev compiled the first version of the periodic table of elements, in which the then-
xxxxxIt was in March 1869 that the Russian chemist Dmitri Mendeleev compiled the first version of the periodic table of elements, in which the then-
xxxxxMendeleev was born in Tobolsk (now Tyumen Oblast), Siberia, and after attending the local school, was trained as a teacher at the Pedagogic Institute in St. Petersburg. On completion of the course he was diagnosed with tuberculosis and, as a result, spent the next two years as a science teacher at Simferopol in the milder climate of the Crimea. Restored to good health, he returned to St. Petersburg in 1857 and, after taking an advanced degree in chemistry, went to Heidelberg in 1859 where he worked on the capillarity of liquids and the development of the spectroscope - tomic weights were to have a marked influence when Mendeleev came to work on his concept of the periodic table.
tomic weights were to have a marked influence when Mendeleev came to work on his concept of the periodic table.
xxxxxHe returned in 1861 and after three years writing on scientific matters, he was appointed Professor of Chemistry at the Technical Institute in St. Petersburg and then succeeded to the chair at the University itself in 1866. Such was the breadth of his knowledge and the brilliance of his teaching, that by 1871 he had made St. Petersburg an internationally recognised centre in this branch of science. And it was there that he came up with his version of the periodic table of elements. Others had produced a classification of elements around this time, such as the English chemist John Newlands (1837-
xxxxxBut Mendeleev was a practical, active man, and his achievements were not confined to theoretical chemistry in the laboratory and lecture hall. In 1867 he was made responsible for the Russian pavilion at the Paris Exposition -
xxxxxFurthermore, outside of his scientific interests, Mendeleev was also active in politics, so much so that his progressive views and his support of a student rebellion in 1890 led to his retirement from St. Petersburg University in 1890. He never held another academic appointment, but three years later he was put in charge of the government’s Bureau of Weights and Measures, a post he filled with distinction until his death in 1907. In his later years honours were heaped upon him from universities and scientific institutions around the world, and in 1905 he was awarded the Copley Medal, the highest award of the Royal Society of London. He was one of the founder members of the Russian Chemical Society in 1869 but, because of his divorce in 1882, and the controversy that surrounded it, he was never admitted to the Russian Academy. Just a few months before his death he was nominated for the Nobel Prize in Chemistry, but failed to be elected by one vote.
xxxxxIncidentally, since Mendeleev’s time, over 40 more chemical elements have been added to the table. The first one discovered in 1955 (element 101) was named “mendelevium” after him, as was a crater on the moon. ……

xxxxx…… When working at the Bureau of Weights and Measures he was tasked with the job of providing a new government standard for the popular Russian drink vodka. He found the perfect alcoholic content was 38%, but the government decided to round it up to 40%. Mendeleev is also credited with introducing the metric system into the Russian Empire. ……
xxxxx…… Through his work, Mendeleev was a close friend of the chemist-
Including:
William Crookes

Vb-
xxxxxAnother scientist at this time who won a name for himself both as a chemist and physicist was the Englishman William Crookes (1832-
 xxxxxAnother scientist at this time who won a name for himself both as a chemist and physicist was the Englishman William Crookes (1832-
xxxxxAnother scientist at this time who won a name for himself both as a chemist and physicist was the Englishman William Crookes (1832-
xxxxxHe was born in London and, after studying at the Royal College of Chemistry, set up his own research laboratory. It was here, in 1875, that he invented the radiometer, a device providing rotary motion which, driven by natural or artificial light, measured the intensity of electromagnetic radiation. And it was during the 1870s that, while attempting to determine the weight of thallium in an evacuated chamber, he extended his research into the conduction of electricity in gases, and produced his most important contribution to physics. Using a 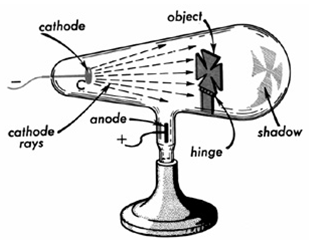 variety of superior vacuum tubes -
variety of superior vacuum tubes -

xxxxxA major contribution to scientific advance was provided by his weekly Chemical News, which he founded in 1859 and edited until 1906. This publication analysed a wide variety of subjects, including the production of sugar beet, the origin of diamonds in South Africa, the dyeing and printing of textiles, the treatment of sewage, the importance of phenol as an antiseptic, and the manufacture and use of artificial fertilizers. It proved so successful that in 1864 he was appointed editor of the Quarterly Journal of Science. He also published papers on spiritualism, a subject which he studied in depth over a number of years. For his valuable services to science, Crookes was knighted in 1897 and received the Order of Merit in 1910.


